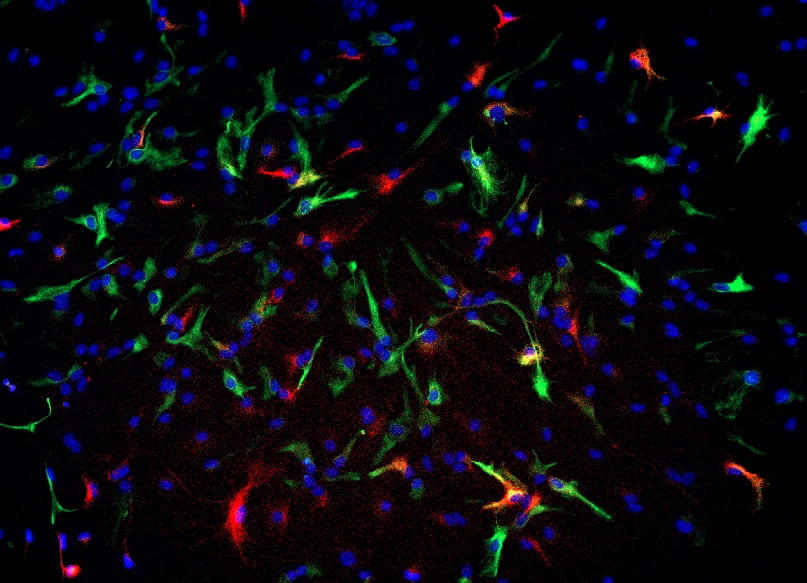
Low-grade gliomas can arise anywhere in the pediatric brain, but tumor location may affect relapse rate and acquisition of neurologic deficits. These tumors are typically driven by mutations activating the MAPK signaling pathway, most frequently the KIAA-1549 BRAF fusion mutation. Notably, incompletely resected tumors in the supratentorial midline region (optic pathway, hypothalamus, thalamus) have a higher rate of serial relapse than incompletely resected tumors elsewhere in the brain, and also a higher incidence of non-BRAF-fusion mutations. We are utilizing a combination of in vitro and in vivo applications to determine how brain location affects NSC response to different oncogenic mutations, the molecular pathways underlying these differences, and whether this correlates with risk of brain tumor formation.
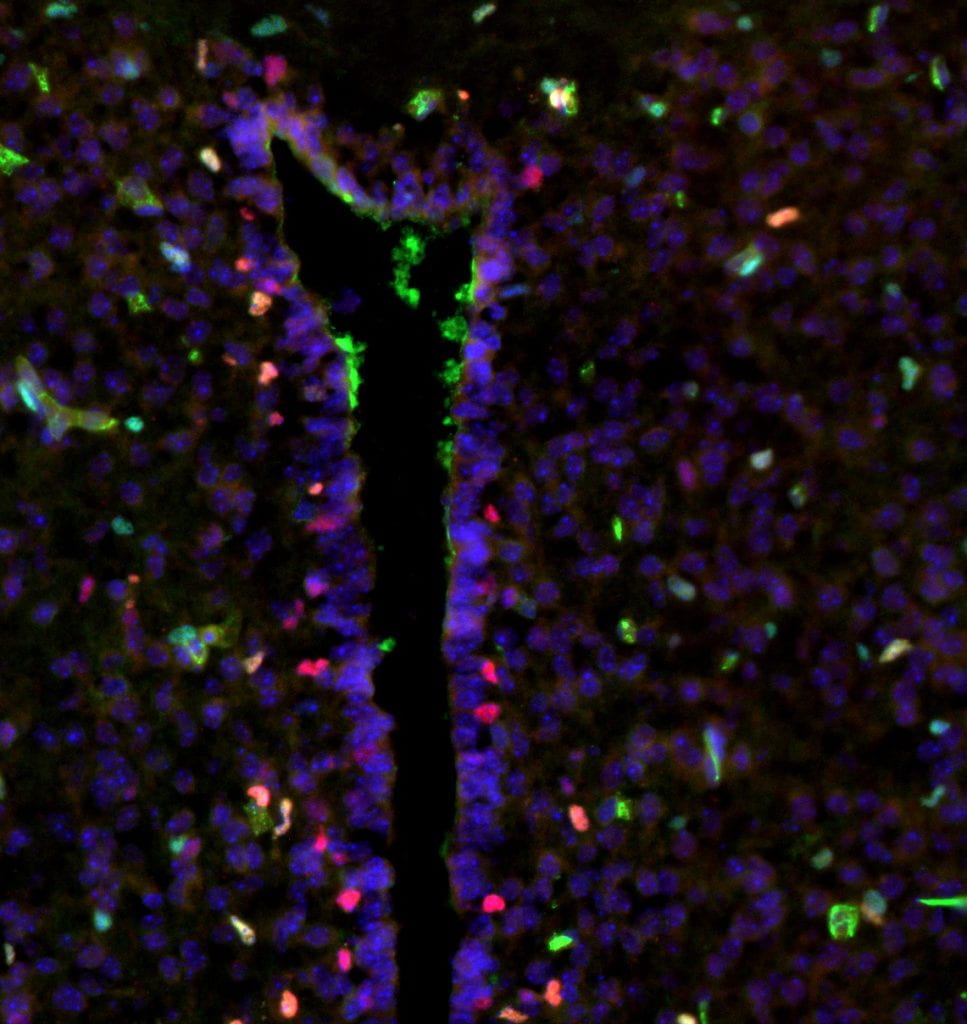
Children of mothers with pre-pregnancy obesity or excessive gestational weight gain have increased rates of pediatric brain tumor formation. Obesity and gestational weight gain are driven in part by diet, and maternal diet-induced obesity models show abnormal development of the fetal brain, particularly near the third ventricular zone (TVZ; location of the cell of origin of NF1-optic pathway glioma). The Brossier lab has demonstrated that obesogenic diet exposure 1) increases proliferation and glial differentiation of the Nf1-OPG cell of origin during embryonic development, and 2) accelerates optic pathway glioma formation and increases tumor penetrance in Nf1-OPG models. Current work focuses on identifying the mechanism by which this occurs, with an eye towards using this information to design risk-adapted treatment strategies for patients with NF1.
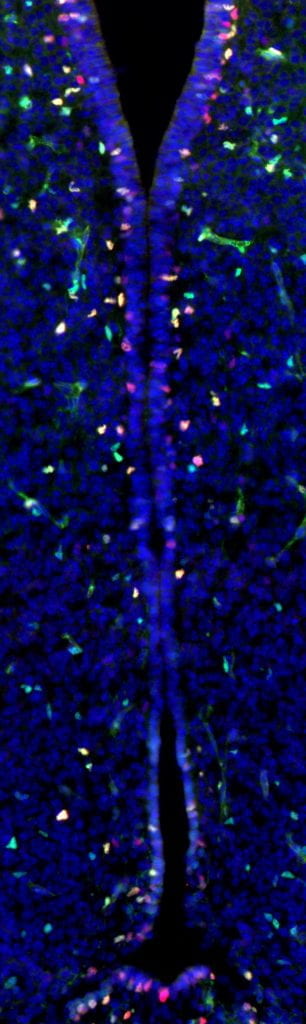
In collaboration with Dr. Yuan Pan (Assistant Professor, UT MD Anderson Cancer Center), the Brossier lab was recently funded to investigate how maternal environmental exposure might modulate cognitive phenotypes in NF1. Together, Drs. Pan and Brossier will study how NF1 mutation and maternal obesogenic diet exposure, both individually and in combination, affect neurodevelopment relative to learning and cognition. This research will provide the foundation for future investigations into risk-adapted therapies for improving cognition in people living with NF1.